About Us
Courage Therapeutics is a leading neuroendocrine company co-founded by Dr. Roger Cone, a pioneering researcher in melanocortin receptor biology and the central melanocortin system’s neural circuits in the brain. The company is dedicated to translating Dr. Cone’s decades of work and discoveries of novel biological mechanisms of energy homeostasis into novel therapeutic candidates targeting the MC4R and MC3R receptors.
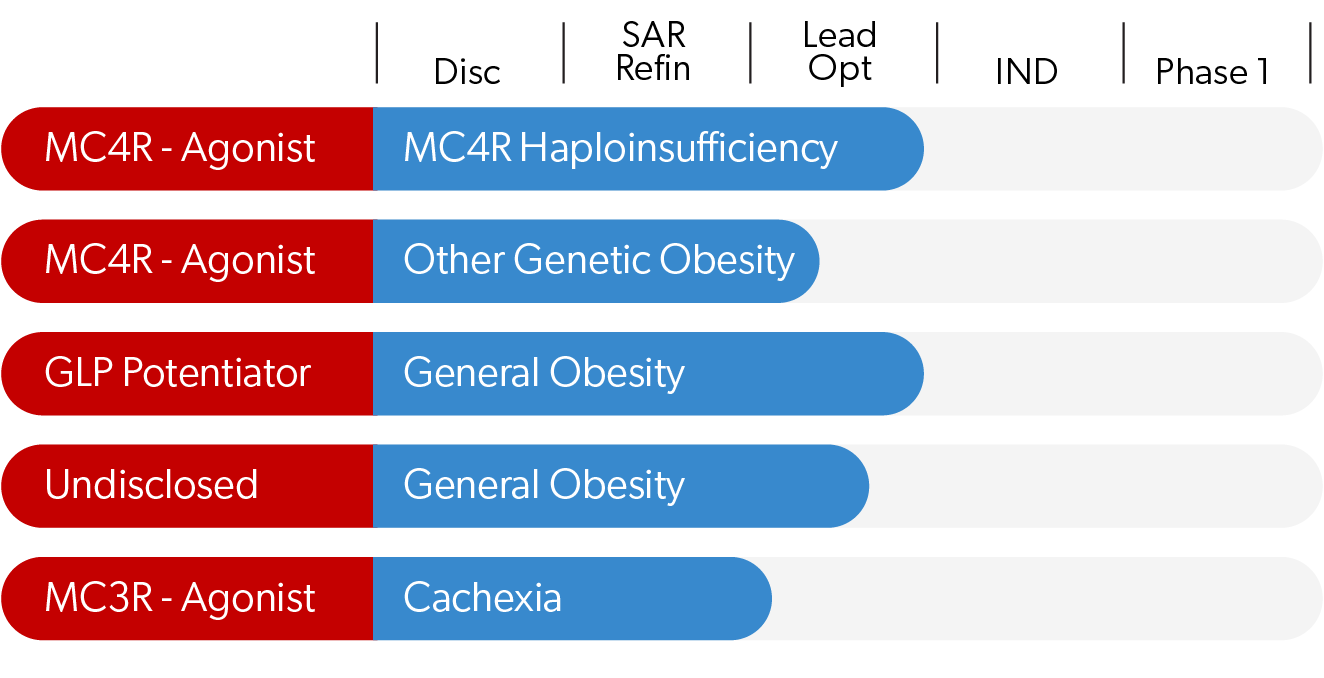
Our Focus
Our MC4R programs focus on treating various genetic obesities as well as addressing dietary obesity in the general population. Activity in various animal models show our compounds have profound monotherapy activity and can also act synergistically with GLP-1s to potently augment their activity and break through their weight loss plateau. Our MC3R program focuses on stimulating food intake with applications in sarcopenia, anorexias and cachexia. Courage Therapeutics is dedicated to developing promising new medicines for these life-threatening diseases.
MC4R Pathway
MC4R is a genetic driver of obesity. Through rectifying insufficient activity of the MC4R pathway that is defective in patients with a number of different genetic obesities, we can correct the genetic defects and augment activity to restore balance and energy homeostasis.
For the broader population suffering from dietary obesity, we seek to overcome challenges for patients where the currently available GL-1 drugs cannot be tolerated, or provide inadequate weight loss for optimal health. Further, muscle mass loss and weight regain upon discontinuation are serious drawbacks for this widespread class of drugs. Our initial results in animals demostrate that when combined with GLP-1s, we achieve more profound weight loss while sparing lean muscle mass and also delaying weight re-gain upon discontinuation of treatment.
MC3R Pathway
Dr. Cone and colleagues have demonstrated that MC3R agonists stimulate food intake, as as also reduce anxiety, suggesting novel utility in multiple eating disorders. We are targeting MC3R to develop therapeutics that have applications in a variety of indications ranging from diseases of wasting such as sarcopenia and cachexia, to more serious disorders such as anorexia nervosa, anorexia of aging and failure-to-thrive in infants.
Leadership
Giovanni Ferrara, M.Sc., M.B.A.
CEO
Roger Cone, Ph.D.
Co-Founder
Tomi Sawyer, Ph.D.
Chief Medi-chem
Sasha Blaug, Ph.D., M.B.A.
CBO-CFO
George Grass, Pharm.D., M.S., Ph.D.
Head of Non-Clinical
Giovanni Ferrara, M.Sc., M.B.A.
CEO

Mr. Ferrara was was most recently President and CEO of Medeor Therapeutics, Inc., a transplant immunology company where he completed the world’s first multi-center, international Phase 3 clinical study that successfully induced immunotolerance in transplant patients allowing them to eliminate the use of toxic immunosuppressive drugs. Prior to that, he was Founder, President and CEO of NeuroVia, Incl, a clinical stage orphan CNS disease company, where he raised a Series A financing, built an efficient team and established the clinical and regulatory strategy to lead the company into an efficacy study. Previously, he was an Entrepreneur-in-Residence at Novartis Venture FundS (NVF), where he founded NeuroVia in 2013 amongst his other activities as an operating partner. He joined NVF from portfolio company Sorbent Therapeutics where he was consulting Chief Business Officer, raising $58M in oversubscribed financing and lead the refocusing of the company’s lead program. Prior roles include General Partner and Managing Director at Burrill & Company after starting his venture capital and business development career at GeneChem Management (including CEO of portfolio company Targanta Therapeutics) and BioChem Pharma. He received his MBA and MSc (molecular endorcrinology) from McGill University.
Roger Cone, Ph.D.
Co-Founder

Roger Cone joined the University of Michigan as 2016 as the Director of the Life Sciences Institute, and was appointed Vice Provost for the Biosciences in 2017. Prior to Michigan, Cone was Professor and Chairman of the Department of Molecular Physiology and Biophysics at Vanderbilt University from 2008-2016. Cone is credited with the discovery of multiple fundamental biological roles for the melanocortin system in energy homeostasis. These findings resulted from studies cloning and characterizing the five receptors for the melanocortin peptides, and analyzing the pharmacological and physiological functions of these receptors. Cone’s group provided the genetic and pharmacological validation of the melanocortin-4 and melanocortin-3 receptors as critical regulators of energy homeostasis, leading to the discovery of mutations in the MC4R as the leading cause of syndromic obesity, and development of the first drug for syndromic obesity, the MC4R agonist Imcivree, approved by the FDA in 2020. Cone has been elected to the National Academy of Sciences (2010), and the National Academy of Medicine (2016) for his work, and received numerous awards, including the Berson Award, Berthold Memorial Award, Ipsen Prize, and the Rolf Luft Prize.
Tomi Sawyer, Ph.D.
Chief Med-chem

Dr. Sawyer is head of drug discovery at Courage. He is an accomplished drug hunter with a track record in both peptide and small molecules. He is the inventor of the MC1R agonist NDP-MSH (Scenesse® by Clinuvel) that was recently approved by the FDA. He also is the inventor of the drugs ALRN-6924 (Aileron) and Iclusig® (Ariad/Takeda). His pharma/biotech career includes being Distinguished Scientist (Merck), CSO (Aileron), SVP (Ariad) and Sr. Director (Pfizer). Tomi has recently founded a worldwide Peptide Drug Hunter Consortium and was a past-president of the American Peptide Society.
Sasha Blaug, Ph.D., M.B.A.
CBO-CFO

Dr Blaug has over 15 years of broad biotech and life science experience as a biotech executive, banker, scientist and investor. Over the course of his career, Sasha has helped build companies in the US and Asia via strategic transactions and negotiations including public and private capital markets transactions, joint ventures, licensing, syndicate formation. Sasha was a biotechnology equity analyst and the equity and convertible debt capital markets healthcare analyst at Piper and Nomura Securities, respectively, and invested in and helped build healthcare companies via his roles at Portola Capital, Lux Capital, Jane Investments, and Nomura Securities. In these roles, Dr. Blaug worked with portfolio companies on strategic initiatives including corporate development and partnerships, portfolio strategy, equity offerings and investor relations, and financial planning and analysis. Most recently, Dr. Blaug was the SVP of corporate development at Biomea Fusion, helping to take the company from a co-founder start-up to a publicly traded biotech, where he positioned the company and helped execute capital markets transactions, and lead the corporate development strategic process, partnership interaction, discussion and negotiations. Prior to his professional career, he wrote for his PhD in Molecular and Cell Biology at UC Berkeley and read for his MBA at Berkeley and Columbia University.
George Grass, Pharm.D., M.S., Ph.D.
Head of Non-Clinical

Dr. George Grass is an accomplished pharmaceutical executive and scientist with extensive experience leading preclinical and translational development across multiple therapeutic areas. As Head of Preclinical Development at Courage Therapeutics, he directs strategy and execution of IND-enabling programs, to advance Courage’s pipeline toward clinical proof of concept.
Before joining Courage, Dr. Grass founded G2 Research Inc. and advised numerous biotech and pharmaceutical organizations on preclinical development, due diligence, and asset strategy and has extensive experience as an expert witness for pharmaceutical patent cases. He has been a founder of NaviCyte, Inc., Precision Instrument Design, Inc., and PDxRx Inc. He has served in senior R&D and corporate leadership roles at multiple venture-backed companies, including Trega Biosciences, Sorbent Therapeutics, and NeuroVia, guiding assets through IND-enabling studies and clinical entry.
Dr. Grass holds Pharm.D., M.S., and Ph.D. degrees in Pharmaceutics and has authored numerous publications and textbook chapters in biopharmaceutics and pharmacokinetics. He combines scientific depth with entrepreneurial insight in transforming early-stage science into clinical and commercial opportunity
Board of Directors
Issac Barchas, J.D.
GP Arsenal Bridge Ventures
Roger Cone, Ph.D.
Co-founder, CSO Courage
Giovanni Ferrara, M.Sc., M.B.A.
CEO Courage
Dan Housman, B.S.
Co-founder Courage
Bruce Leuchter, M.D.
CEO Neurvati
Issac Barchas, J.D.
GP Arsenal Bridge Ventures

Isaac Barchas is a founding partner of Arsenal Bridge Ventures. He previously served as CEO of Research Bridge Partners, which he co-founded with Reid Hoffman, and continues to serve as a special advisor to the company. Barchas is a managing director of Research Bridge Partners’ Catalyst Fund and Arsenal Bridge Ventures II, II-B, III, and IV. He also served as founding board member of Tetricus, Eradivir, and MorphImmune (NASDAQ: IMNM) and as lead turnaround board member of Novosteo (NASDAQ: QNCX) and Courage Therapeutics. Prior to founding Research Bridge Partners, Barchas led the Austin Technology Incubator where he raised ~$1B in investor capital and, before that, was a leader in McKinsey & Co.’s healthcare and organization practices.
Roger Cone, Ph.D.
Co-founder, CSO Courage

Roger Cone joined the University of Michigan as 2016 as the Director of the Life Sciences Institute, and was appointed Vice Provost for the Biosciences in 2017. Prior to Michigan, Cone was Professor and Chairman of the Department of Molecular Physiology and Biophysics at Vanderbilt University from 2008-2016. Cone is credited with the discovery of multiple fundamental biological roles for the melanocortin system in energy homeostasis. These findings resulted from studies cloning and characterizing the five receptors for the melanocortin peptides, and analyzing the pharmacological and physiological functions of these receptors. Cone’s group provided the genetic and pharmacological validation of the melanocortin-4 and melanocortin-3 receptors as critical regulators of energy homeostasis, leading to the discovery of mutations in the MC4R as the leading cause of syndromic obesity, and development of the first drug for syndromic obesity, the MC4R agonist Imcivree, approved by the FDA in 2020. Cone has been elected to the National Academy of Sciences (2010), and the National Academy of Medicine (2016) for his work, and received numerous awards, including the Berson Award, Berthold Memorial Award, Ipsen Prize, and the Rolf Luft Prize.
Giovanni Ferrara, M.Sc., M.B.A.
CEO Courage

Mr. Ferrara was was most recently President and CEO of Medeor Therapeutics, Inc., a transplant immunology company where he completed the world’s first multi-center, international Phase 3 clinical study that successfully induced immunotolerance in transplant patients allowing them to eliminate the use of toxic immunosuppressive drugs. Prior to that, he was Founder, President and CEO of NeuroVia, Incl, a clinical stage orphan CNS disease company, where he raised a Series A financing, built an efficient team and established the clinical and regulatory strategy to lead the company into an efficacy study. Previously, he was an Entrepreneur-in-Residence at Novartis Venture FundS (NVF), where he founded NeuroVia in 2013 amongst his other activities as an operating partner. He joined NVF from portfolio company Sorbent Therapeutics where he was consulting Chief Business Officer, raising $58M in oversubscribed financing and lead the refocusing of the company’s lead program. Prior roles include General Partner and Managing Director at Burrill & Company after starting his venture capital and business development career at GeneChem Management (including CEO of portfolio company Targanta Therapeutics) and BioChem Pharma. He received his MBA and MSc (molecular endorcrinology) from McGill University.
Dan Housman, B.S.
Co-founder Courage

Dan is a serial entrepreneur who founded and led multiple companies in the healthcare technology space. His start-up Recombinant Data Corp. ["RDC"] focused on analytics on genomics and patient data to support translational research. Following the acquisition of RDC by Deloitte he remained at the company as a Managing Director of the Healthcare and Life Sciences practice leading initiatives around life sciences uses of analytics and AI for drug discovery and development. He holds a BS in Chemistry and Biology from MIT and continues to mentor entrepreneurs through the MIT VMS entrepreneurship service. He is a parent of daughter with Anorexia Nervosa and son with ARFID.
Bruce Leuchter, M.D.
CEO Neurvati

Dr. Bruce Leuchter is President, CEO, and board member of Neurvati Neurosciences and GRIN Therapeutics. A physician by training and neuropsychiatrist by specialty, Dr. Leuchter served in senior roles for over a decade in biotechnology equity research at Goldman Sachs, healthcare investment banking at Credit Suisse, and mergers and acquisitions at PJT Partners, where he provided strategic and capital markets advisory to leading biopharmaceutical companies.
Dr. Leuchter served as the Director of Clinical Neuropsychiatry at Weill Cornell Medical College, where he currently holds a voluntary faculty appointment. He is a co-founder and the founding neuropsychiatrist of Click Therapeutics. He serves on the board of Arrivo BioVentures, is a member of the Scientific Advisory Committee for the Daedalus Fund for Innovation at Weill Cornell, and sits on the Life Sciences Institute Leadership Council at the University of Michigan.
Recent News
November 2025
Courage Therapeutics Completes Seed Financing to Advance Its Melanocortin Obesity and Diseases of Wasting Drug Programs
May 2025
U-M startup Courage Therapeutics receives $7.8M investment to advance weight regulation drugs
July 2024
Courage MC4R agonist drug candidates demonstrate potential to transform GLP1 obesity therapy
Publications
July 2024
Subthreshold activation of the melanocortin system causes generalized sensitization to anorectic agents in mice
The Journal of Clinical Investigation
July 2024
New research demonstrates potential for increasing effectiveness of popular diabetes, weight-loss drugs
Michigan News
July 2024
Research Reveals Method to Improve Diabetes and Weight Loss Drug Performance
Medical Dialogues
November 2021
The brain sensor discovery behind humans getting taller
BBC News
November 2021
MC3R links nutritional state to childhood growth and the timing of puberty
Nature
July 2021
Adjusting Proteins Increases Ozempic's Effectiveness
Neuroscience News
April 2021
The melanocortin-3 receptor is a pharmacological target for the regulation of anorexia
Science Translational Medicine
April 2021
Network dynamics of hypothalamic feeding neurons
Proceedings of the National Academy of Sciences
August 2018
Receptor protein in the brain controls the body's fat Rheostat
U. Michigan
August 2018
Regulation of energy rheostasis by the melanocortin-3 receptor
Science Advances
Contact
For more information contact us at: info@couragetx.com